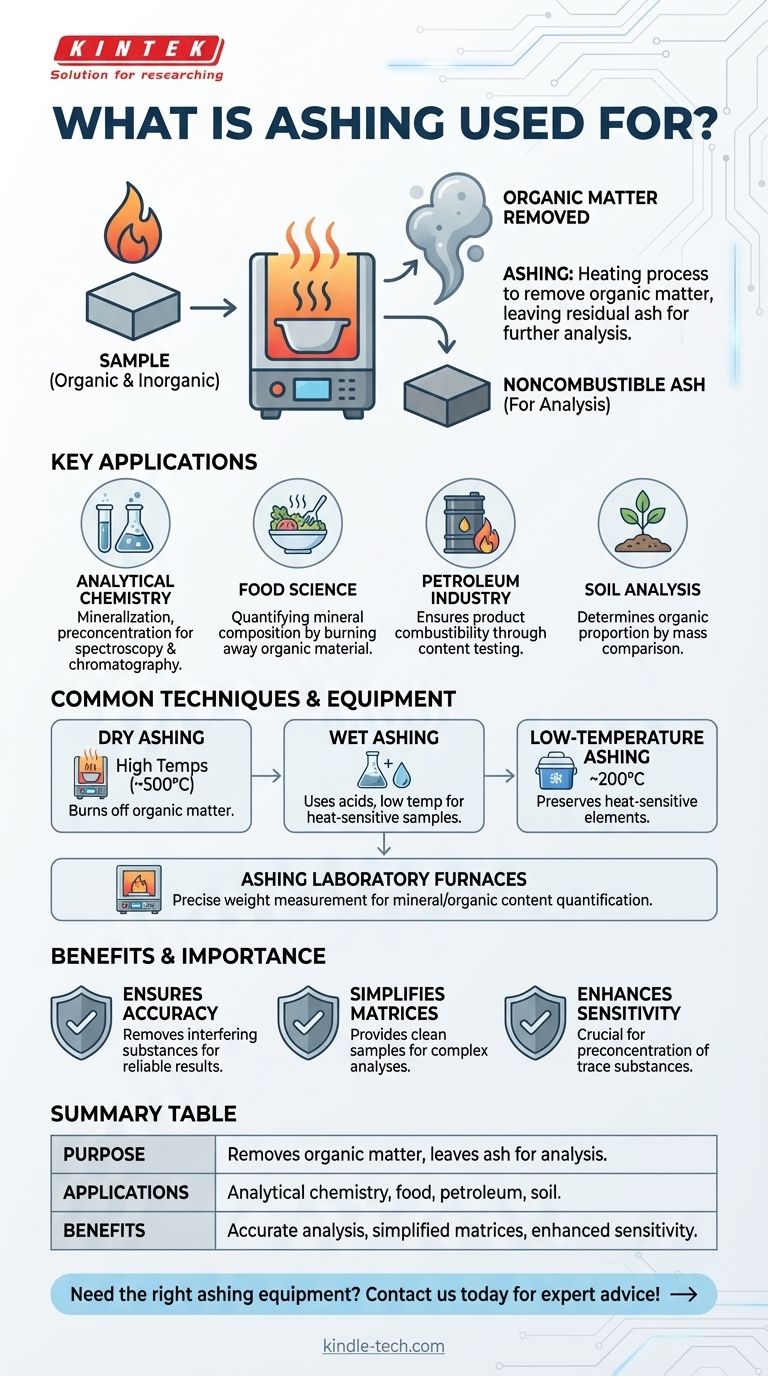
회화(Ashing)는 다양한 산업 및 과학 분야에서 유기물 및 기타 방해 성분을 샘플에서 제거하여 비연소성 재를 남겨 추가 분석을 수행하는 데 사용되는 중요한 공정입니다. 이는 분석 화학, 식품 과학, 석유 테스트 및 토양 분석에 일반적으로 사용됩니다. 건식 회화, 습식 회화 및 저온 회화와 같은 기술은 샘플 유형 및 분석 요구 사항에 따라 사용됩니다. 회화는 미네랄 조성을 정량화하고, 제품의 연소성을 보장하며, 분광학 및 크로마토그래피와 같은 기술을 위한 샘플을 준비하는 데 도움이 됩니다. 또한 미량 물질의 사전 농축 및 토양과 같은 물질의 유기물 비율을 결정하는 데 필수적입니다.

주요 요점 설명:
-
회화의 정의 및 목적
- 회화는 샘플을 가열하여 유기물 및 기타 방해하는 매트릭스 성분을 제거하고 비연소성 재를 남기는 공정입니다.
- 주로 측정에 방해가 될 수 있는 성분을 제거하여 정확한 분석을 위한 샘플을 준비하는 데 사용됩니다.
-
회화의 응용 분야
- 분석 화학: 회화는 크로마토그래피 또는 분광학과 같은 화학적 또는 광학적 분석 전에 미량 물질의 광물화 및 사전 농축에 사용됩니다.
- 식품 과학: 유기물을 태워 식품 샘플의 미네랄 조성을 정량화하는 데 도움이 됩니다.
- 석유 산업: 회화는 석유 제품의 함량을 테스트하여 연소성을 보장합니다.
- 토양 분석: 회화 전후의 질량을 비교하여 토양의 유기물 비율을 결정합니다.
-
일반적인 회화 기술
- 건식 회화: 고온(약 500°C)에서 샘플을 가열하여 유기물을 태워 없앱니다.
- 습식 회화: 고온 대신 산을 사용하여 유기 성분을 제거하며, 열에 민감한 샘플에 적합합니다.
- 저온 회화: 열에 민감한 원소를 보존하기 위해 더 낮은 온도(약 200°C)에서 수행됩니다.
- 황산 회화: 샘플에서 이산화황을 중화하고 제거합니다.
- 밀폐 시스템 회화: 밀폐된 챔버를 사용하여 공정 중 분위기를 제어하여 정밀도를 보장합니다.
-
흑연로 원자 흡수 분광법(AA)에서의 역할
- 회화는 흑연로 AA 프로그램에서 중요한 단계이며, 분석물 측정에 방해가 될 수 있는 매트릭스 성분을 제거합니다.
- 이 단계는 원소 분석에서 정확하고 신뢰할 수 있는 결과를 보장합니다.
-
샘플 준비에서의 중요성
- 회화는 샘플 매트릭스를 단순화하여 잔류 재의 원소 조성을 더 쉽게 분석할 수 있도록 합니다.
- 특히 미량 물질의 사전 농축에 유용하여 분석 기술의 감도를 향상시킵니다.
-
회화에 사용되는 장비
- 회화 실험실 전기로: 유기 성분이 연소될 때 샘플의 무게 변화를 측정하도록 설계되었습니다.
- 이러한 전기로는 미네랄 또는 유기물 함량의 정밀한 정량이 필요한 산업에 필수적입니다.
-
회화의 이점
- 방해 물질을 제거하여 정확한 분석을 보장합니다.
- 분광학 및 크로마토그래피와 같은 기술을 위한 깨끗한 샘플 매트릭스를 제공합니다.
- 다양한 물질의 미네랄 함량 및 유기물 비율을 정량화하는 데 도움이 됩니다.
이러한 주요 요점을 이해함으로써 장비 및 소모품 구매자는 샘플 준비 및 분석에서 회화의 중요성을 더 잘 이해하고 특정 요구 사항에 맞는 올바른 도구와 기술을 선택할 수 있습니다.
요약 표:
| 측면 | 세부 사항 |
|---|---|
| 목적 | 유기물을 제거하고 분석을 위한 비연소성 재를 남깁니다. |
| 응용 분야 | 분석 화학, 식품 과학, 석유 테스트, 토양 분석. |
| 기술 | 건식 회화, 습식 회화, 저온 회화, 황산 회화. |
| 장비 | 정밀한 무게 측정을 위한 회화 실험실 전기로. |
| 이점 | 정확한 분석 보장, 샘플 매트릭스 단순화, 감도 향상. |
실험실에 적합한 회화 장비가 필요하신가요? 지금 문의하세요 전문가의 조언과 솔루션을 제공해 드립니다!
시각적 가이드
관련 제품
- 실험실용 1800℃ 머플로 퍼니스
- 1700℃ 실험실용 머플로 퍼니스
- 실험실 머플로 오븐 퍼니스 하부 리프팅 머플로 퍼니스
- 실험실용 1400℃ 머플 오븐 퍼니스
- 실험실 탈바가지 및 소결 전 가열로



















